Abstract
Background: This systematic review evaluates the efficacy and safety of Mycophenolate Mofetil (MMF) for managing treatment‐resistant Inflammatory Bowel Disease (IBD), emphasizing remission rates and adverse effects.
Methods: Observational and controlled trials assessing MMF’s impact on IBD were included, excluding non-English and pediatric studies. Comprehensive searches were conducted in Embase, Medline/PubMed, Scopus, and Web of Science through October 2023. The risk of bias was evaluated using the NIH quality assessment tool, and results were synthesized using a random-effects meta-analysis model.
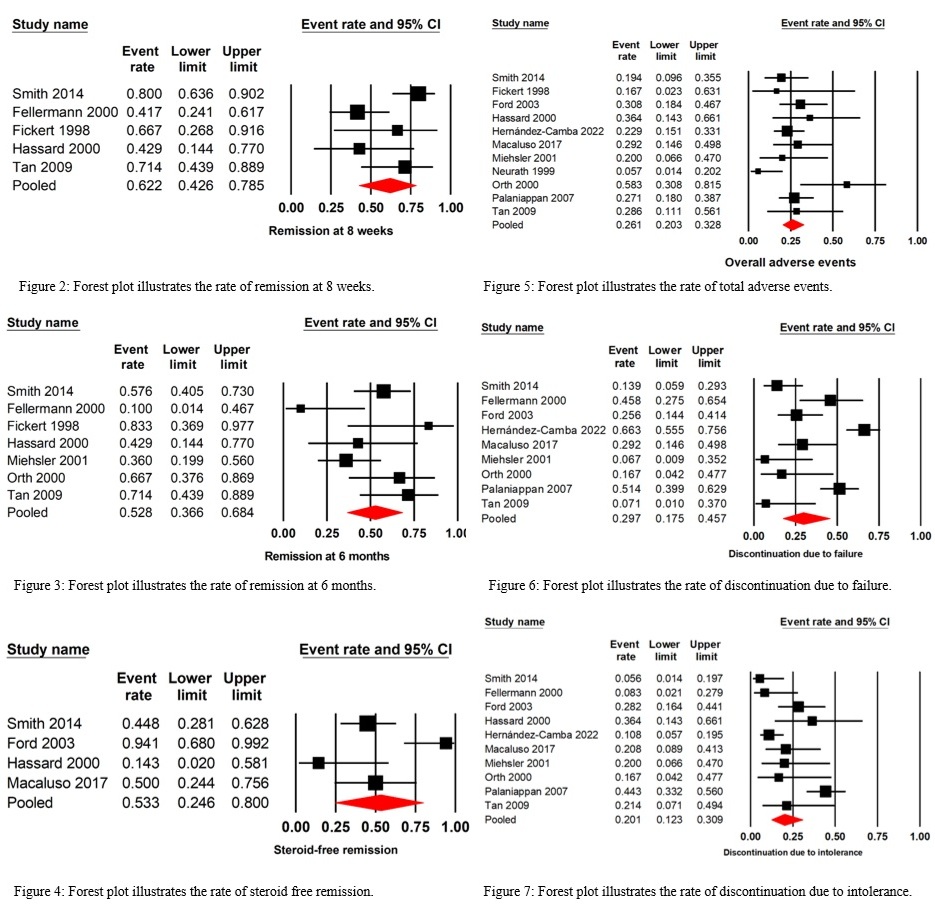
Results: Twelve studies comprising 446 participants (333 with Crohn’s disease and 113 with ulcerative colitis) were analyzed. The meta-analysis revealed remission rates of 62.2% at 8 weeks and 52.8% at 6 months. Adverse effects occurred in 26.1% of patients, with nausea and vomiting being the most common. Treatment discontinuation due to failure and intolerance was observed in 29.7% and 20% of cases, respectively.
Discussion: The findings suggest that MMF effectively induces remission in IBD patients unresponsive to conventional therapies, although a notable proportion experienced adverse events or treatment failure. Careful patient selection and monitoring are essential.
Conclusion: MMF presents a promising alternative for managing resistant IBD, but its adverse effect profile warrants cautious application. Further research is needed to optimize dosing strategies and assess long-term outcomes in this challenging patient population. These results underscore the potential of MMF as an effective therapeutic option while emphasizing the importance of individualized treatment plans and rigorous clinical monitoring. Future studies should focus on long-term safety and dosing. Additional robust research is required.
References
1. Zhang YZ, Li YY. Inflammatory bowel disease: pathogenesis. World J Gastroenterol. 2014: 91 [PMID: 24415861 https://doi.org/10.3748/wjg.v20.i1.91]
2. Moazzami B, Moazzami K, Rezaei N. Early onset inflammatory bowel disease: manifestations, genetics and diagnosis. Turk J Pediatr. 2019: 637 [PMID: 32104994 https://doi.org/10.24953/turkjped.2019.05.001]
3. Rodrigues BL, Mazzaro MC, Nagasako CK, Ayrizono MLS, Fagundes JJ, Leal RF. Assessment of disease activity in inflammatory bowel diseases: Non-invasive biomarkers and endoscopic scores. World J Gastrointest Endosc. 2020: 504 [PMID: 33362904 https://doi.org/10.4253/wjge.v12.i12.504]
4. Rothfuss KS, Stange EF, Herrlinger KR. Extraintestinal manifestations and complications in inflammatory bowel diseases. World J Gastroenterol. 2006: 4819 [PMID: 16937463 https://doi.org/10.3748/wjg.v12.i30.4819]
5. Kim ER, Chang DK. Colorectal cancer in inflammatory bowel disease: the risk, pathogenesis, prevention and diagnosis. World J Gastroenterol. 2014: 9872 [PMID: 25110418 https://doi.org/10.3748/wjg.v20.i29.9872]
6. Chakrabarti K, Frame D, Al Abbas M, McCune WJ. The use of mycophenolate mofetil area under the curve. Curr Opin Rheumatol. 2021: 221 [PMID: 33741807 https://doi.org/10.1097/BOR.0000000000000799]
7. Orvis AK, Wesson SK, Breza TS, Jr., Church AA, Mitchell CL, Watkins SW. Mycophenolate mofetil in dermatology. J Am Acad Dermatol. 2009: 183 [PMID: 19150270 https://doi.org/10.1016/j.jaad.2008.08.049]
8. Surjushe A, Saple DG. Mycophenolate mofetil. Indian J Dermatol Venereol Leprol. 2008: 180 [PMID: 18388396 https://doi.org/10.4103/0378-6323.39725]
9. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010: 336 [PMID: 20171303 https://doi.org/10.1016/j.ijsu.2010.02.007]
10. Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, Thomas J. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019: ED000142 [PMID: 31643080 https://doi.org/10.1002/14651858.ED000142]
11. Murray A, Nguyen TM, Parker CE, Feagan BG, MacDonald JK. Oral 5-aminosalicylic acid for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2020: CD000543 [PMID: 32786164 https://doi.org/10.1002/14651858.CD000543.pub5]
12. Bruscoli S, Febo M, Riccardi C, Migliorati G. Glucocorticoid Therapy in Inflammatory Bowel Disease: Mechanisms and Clinical Practice. Front Immunol. 2021: 691480 [PMID: 34149734 https://doi.org/10.3389/fimmu.2021.691480]
13. Jeong DY, Kim S, Son MJ, Son CY, Kim JY, Kronbichler A, Lee KH, Shin JI. Induction and maintenance treatment of inflammatory bowel disease: A comprehensive review. Autoimmun Rev. 2019: 439 [PMID: 30844556 https://doi.org/10.1016/j.autrev.2019.03.002]
14. Lee LYW, Gardezi AS, Santharam V, Boyd J, Lanzon-Miller S. Effect of azathioprine intolerance on outcomes of inflammatory bowel disease: a cross-sectional study. Frontline Gastroenterol. 2014: 40 [PMID: 28839749 https://doi.org/10.1136/flgastro-2013-100348]
15. Hassard PV, Vasiliauskas EA, Kam LY, Targan SR, Abreu MT. Efficacy of mycophenolate mofetil in patients failing 6-mercaptopurine or azathioprine therapy for Crohn's disease. Inflamm Bowel Dis. 2000: 16 [PMID: 10701145 https://doi.org/10.1097/00054725-200002000-00003]
16. Ford AC, Towler RJ, Moayyedi P, Chalmers DM, Axon AT. Mycophenolate mofetil in refractory inflammatory bowel disease. Aliment Pharmacol Ther. 2003: 1365 [PMID: 12786630 https://doi.org/10.1046/j.1365-2036.2003.01581.x]
17. Fickert P, Hinterleitner TA, Wenzl HH, Aichbichler BW, Petritsch W. Mycophenolate mofetil in patients with Crohn's disease. Am J Gastroenterol. 1998: 2529 [PMID: 9860419 https://doi.org/10.1111/j.1572-0241.1998.00606.x]
18. Miehsler W, Reinisch W, Moser G, Gangl A, Vogelsang H. Is mycophenolate mofetil an effective alternative in azathioprine-intolerant patients with chronic active Crohn's disease? Am J Gastroenterol. 2001: 782 [PMID: 11280551 https://doi.org/10.1111/j.1572-0241.2001.03622.x]
19. Fellermann K, Steffen M, Stein J, Raedler A, Hamling J, Ludwig D, Loeschke K, Stange EF. Mycophenolate mofetil: lack of efficacy in chronic active inflammatory bowel disease. Aliment Pharmacol Ther. 2000: 171 [PMID: 10651657 https://doi.org/10.1046/j.1365-2036.2000.00695.x]
20. Lim SZ, Chua EW. Revisiting the Role of Thiopurines in Inflammatory Bowel Disease Through Pharmacogenomics and Use of Novel Methods for Therapeutic Drug Monitoring. Front Pharmacol. 2018: 1107 [PMID: 30349479 https://doi.org/10.3389/fphar.2018.01107]
21. Velikova T, Sekulovski M, Peshevska-Sekulovska M. Immunogenicity and Loss of Effectiveness of Biologic Therapy for Inflammatory Bowel Disease Patients Due to Anti-Drug Antibody Development. Antibodies (Basel). 2024: [PMID: 38534206 https://doi.org/10.3390/antib13010016]
22. Smith MR, Cooper SC. Mycophenolate mofetil therapy in the management of inflammatory bowel disease--a retrospective case series and review. J Crohns Colitis. 2014: 890 [PMID: 24507162 https://doi.org/10.1016/j.crohns.2014.01.014]
23. Neurath MF, Wanitschke R, Peters M, Krummenauer F, Meyer zum Buschenfelde KH, Schlaak JF. Randomised trial of mycophenolate mofetil versus azathioprine for treatment of chronic active Crohn's disease. Gut. 1999: 625 [PMID: 10205197 https://doi.org/10.1136/gut.44.5.625]
24. Palaniappan S, Ford AC, Greer D, Everett SM, Chalmers DM, Axon AT, Hamlin PJ. Mycophenolate mofetil therapy for refractory inflammatory bowel disease. Inflamm Bowel Dis. 2007: 1488 [PMID: 17924566 https://doi.org/10.1002/ibd.20258]
25. Macaluso FS, Maida M, Renna S, Orlando E, Affronti M, Sapienza C, Dimarco M, Orlando R, Rizzuto G, Cottone M, Orlando A. Mycophenolate mofetil is a valid option in patients with inflammatory bowel disease resistant to TNF-alpha inhibitors and conventional immunosuppressants. Dig Liver Dis. 2017: 157 [PMID: 27876682 https://doi.org/10.1016/j.dld.2016.10.001]
26. Hernandez-Camba A, Arranz L, Vera I, Carpio D, Calafat M, Lucendo AJ, Taxonera C, Marin S, Garcia MJ, Marin GS, Rodriguez ES, Carbajo AY, De Castro ML, Iborra M, Martin-Cardona A, Rodriguez-Lago I, Busquets D, Bertoletti F, Ausin MS, Tardillo C, Malaves JH, Bujanda L, Castano A, Domenech E, Ramos L, Geteccu, Additional member of the Spanish Gg. Real-world use of mycophenolate mofetil in inflammatory bowel disease: Results from the ENEIDA registry. Dig Liver Dis. 2022: 635 [PMID: 34862115 https://doi.org/10.1016/j.dld.2021.10.002]
27. Orth T, Peters M, Schlaak JF, Krummenauer F, Wanitschke R, Mayet WJ, Galle PR, Neurath MF. Mycophenolate mofetil versus azathioprine in patients with chronic active ulcerative colitis: a 12-month pilot study. Am J Gastroenterol. 2000: 1201 [PMID: 10811328 https://doi.org/10.1111/j.1572-0241.2000.02010.x]
28. Tan T, Lawrance IC. Use of mycophenolate mofetil in inflammatory bowel disease. World J Gastroenterol. 2009: 1594 [PMID: 19340901 https://doi.org/10.3748/wjg.15.1594]

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright (c) 2025 Natalie Balassiano, Jasmine Tidwell , Omar Abdelhalim, Mohammed Abusuliman, Mahmoud Nassar, Azizullah Beran, Ivan Cancarevic, Hazem Abosheaishaa